This set of problems is for your benefit, you would be well served by working on these before the first exam!
To encourage you to do so: submisson of a completed problem set to your lecture instructor by Noon on Monday 9/20/98 Those observing Yom Kippur (and any one else that wishes) are encouraged to submit their work early! will result in 5 points extra credit.
Answers will be posted on Monday 9/20/98 by 5 p.m.
1. Draw the electron shells and give the valences of : Hydrogen (H), Carbon (C), Argon (Ar), and Potassium (K)
2. Which of the atoms in #1 is effectively inert and why?
3. For the following atoms, give the atomic number and atomic mass in this notation: Helium = mass 4, number 2
Sodium, potassium, hydrogen, carbon and fluorine.
4. For each of the atoms below, give two possible isotopes.
Hydrogen, carbon and oxygen
5. What happens to potassium and chlorine when they form an ionic bond to give KCl (potasium chloride)
6. Draw the structure of water, in the solid phase, indicating covalent and hydrogen bonds.
7. If a molecule contains all non polar covalent bonds what would expect to happen when that molecule is mixed with water and why?
8. Which properties of water result from the hydrogen bonds between water molecules?
9. For reactant R and product P in a a reversible reaction, must [P] equal [R] at chemical equilibrium? If not, why not?
10. Locate a periodic table and give the molecular weight (= grams/ mole) of
H2CO3
NaCl
H20
C6H1206
CH3OH
H2SO4
11. One mole of any substance dissolved in 1 liter of solution, gives a one molar (M) solution. How many grams of KCl must you dissolve to make a 1 molar solution?
Molecular weight is ~75 g; 75 g/mole X 1 mole/ 1 l liter = 75 g/ liter
How many grams must you dissolve to give a 0.2 molar solution?
0.2 M is 0.2 moles/liter; 0.2 moles/liter X 75 g/mole = 15 g/liter
How many grams must you dissolve to give a 3 mM solution?
3 mM = 3 milimoles/liter X mole/ 1000 milimoles X 75 g/ mole = 0.225 g/liter
Calculate, using the examples above, the number of grams you would have to weigh out per liter of solution to give a:
1 M solution of NaCl
A 0.2 mM solution of NaCl
A 5 M solution of C6H1206
A 11 mM solution of C6H1206
A 25 mM solution of MgCl2
A 0.3 M solution of MgCl2
12. How many molecules are there in 1 liter of a 1 Molar solution of any compound?
1M = 1mole/1liter X 6.02 X1023 molecules/mole = 6.02 X1023 molecules/liter.
How many molecules are there in 10g of NaCl?
10g X mole/59 g X 6.02 X1023 molecules/mole = 1.02 X1023 molecules.
Using the examples above, complete the following problems:
How many molecules are there in:
50 g of KCl ?
20 g of H20 ?
One liter of a 5M solution of HCl (HCl molecules only, please) ?
13. A solution has a pH of 4, what is the hydrogen (hydronium) ion concentration?
14. A solution has a hydrogen ion concentration of 1 X10-11 M what is the pH?
Is this an acidic or a basic solution?
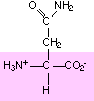
15. Circle and name the ALL functional groups on these molecules:

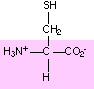
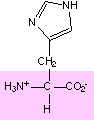
These images came from a Biochemistry site at UC Davis . Check it out!
16. List all the types of bonds in order of decreasing strength.
17. Show how a peptide bond forms between two amino acids.
18. Draw a phospholipid, lipid, a nucleotide, two different monsaccharides, and three different amino acids.