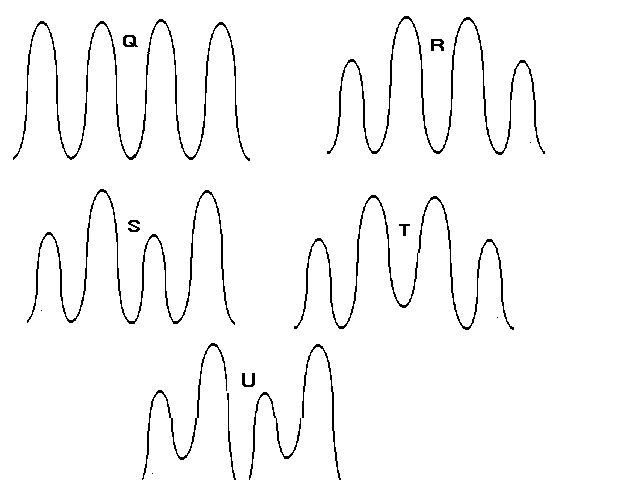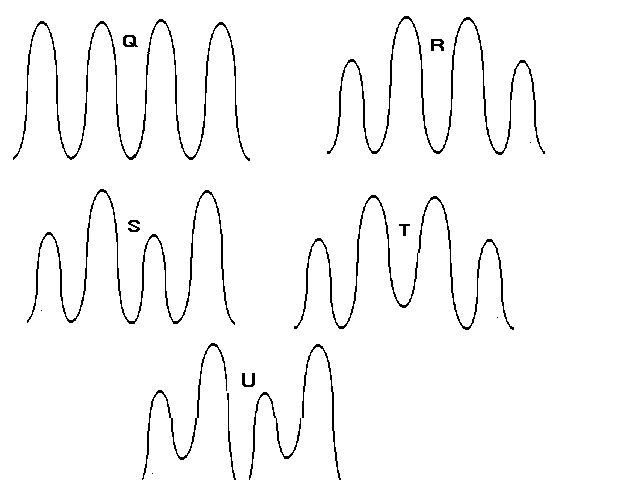Sorry, but answer a is not the right answer.
Please try again. |
Answer b is incorrect.
Please read the question again and make another choice. |
No, c is a wrong answer.
Please repeat this question. |
This answer, d, is incorrect.
Please review this question and try again. |
Answer e is a wrong answer.
Please repeat this question. |
Yes! That's the right answer!
Now move to the next question. |
Right! You chose the correct answer!
Continue with the next question. |
You are right!
Move along to the next question. |
Good choice! That's the right answer!
Now for the next question. |
You picked the correct answer!
Continue to the next question. |
Please note: If you are not familiar with these fileexaminations, you can learn how to work with them in apractice examination.
You can leave this quiz and return to the file examination menupage at any time by moving to the bottom andfollowing the link provided.
| CHM 201 E | -- | Examination I | -- | 27 Sep 1999 |
This examination comprises 33 questions.
01. In geometric shape, a methane molecule is:
- a linear
- b tetrahedral
- c a planar square
- d an equilateral triangle
- e cubical
02. The IUPAC name of isobutyl bromide is:- a 2-bromo-1-methylpropane
- b 2-bromobutane
- c 2-bromo-2-methylpropane
- d 1-bromo-2-methylpropane
- e 1-bromo-2-methylbutane
03. Isopentane is an isomer of:- a 2,2-dimethylpropane
- b 2,3-dimethylpropane
- c isobutane
- d 2-methylbutane
- e 2,3-dimethylbutane
04. Of the following, an incorrect IUPAC nameis:- a 3-methylhexane
- b 3-ethyl-2-methyloctane
- c 1,2,4-trimethylcyclopentane
- d 2,5-diethylnonane
- e ethylcyclopropane
05. The pair of constitutional isomers among thefollowing is:- a staggered propane and eclipsedpropane
- b cis-2-butene and trans-2-butene
- c axial-methylcyclohexane andequatorial-methylcyclohexane
- d cyclopentane andmethylcyclobutane
- e isobutane and neopentane
06. The IUPAC name of CH3-CH2-CH2 | CH3-CH2
is:- a 1-ethylpropane
- b 1-methylbutane
- c pentane
- d 1-propylethane
- e 1-ethyl-2-methylethane
07. The IUPAC name of CH3-CH2 | CH3-CH2-C-CH3 | CH2 | CH3-CH-CH3
is: - a 2-ethyl-2-isobutylbutane
- b 2-ethyl-2-methyl-1-isopropylbutane
- c 3-ethyl-3,5-dimethylhexane
- d 4-ethyl-2,4-dimethylhexane
- e 2-ethyl-1,2,4-trimethylpentane
08. The compound among the following that hasone or moretertiary hydrogens is: - a 2,2,3,3-tetramethylbutane
- b cyclopentane
- c neopentane
- d butane
- e methylcyclohexane
09. Covalent bond formation between an ethylfree radical and atert-butyl free radical produces:- a isobutane
- b isopentane
- c 2,2-dimethylbutane
- d 2,3-dimethylbutane
- e neopentane
10. The molecular formulaC8H16 cannot represent:- a a branched-chain compound
- b a cycloalkane
- c a straight-chainalkane
- d a trans alkene
- e one of the isomers ofdimethylcyclohexane
11. A name that is not a correct IUPAC name forone of theisomers of C4H9Br is:- a 1-bromo-2-methylpropane
- b 1-bromo-1-methylpropane
- c 2-bromobutane
- d 2-bromo-2-methylpropane
- e 1-bromobutane
12. The IUPAC name of CH2 / \ CH2 CH-CH3 | | CH2 CH2 \ / C / \ CH3 CH3
is:- a 2,4,4-trimethylcyclohexane
- b 1,3,3-trimethylcyclohexane
- c 1,1,3-trimethylcyclohexane
- d 2-methyl-4,4-dimethylcyclohexane
- e 1-methyl-3,3-dimethylcyclohexne
USE THE FOLLOWING GRAPHIC FOR QUESTIONS 13 AND14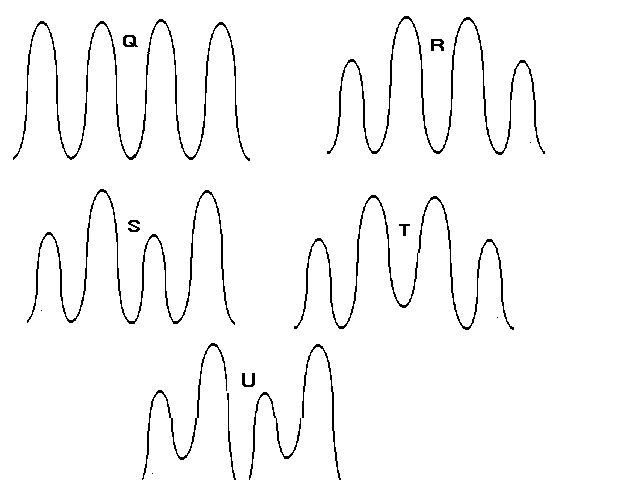
13. Which one of the curves represents a portion of the potentialenergy vs. bond rotation graph for rotation about the C1-C2 bondin 2-methylbutane,
CH3-CH-CH2-CH3 | CH3
:14. Which one of the curves represents a portionof the potential energy vs. bond rotation graph for rotationabout the C2-C3 bond in 2-methylbutane, CH3-CH-CH2-CH3 | CH3
: 15. Adolph von Baeyer reasoned (incorrectly)that of all thecycloalkanes, cyclopentane should show the smallest ring-strainenergy because:- a its internal C-C-Cangles deviate least from the tetrahedralangle
- b it has the smallest number ofC/C eclipsing interactions
- c it has the smallest number ofH/H eclipsing interactions
- d its external H-C-H anglesdeviate least from the tetrahedralangle
- e its heat of combustion, per CH2group, is the smallest for all the cycloalkanes
16. In actual fact, of all the cycloalkanes it iscyclohexane that shows the smallest ring-strain energy. This isbecause:- a its carbon atoms are nearlysp2 hybridized
- b hexagons have the lowest energyof all geometric figures
- c C-C bond lengths are shortestin cyclohexane
- d C-C bond angles are longest incyclohexane
- e the cyclohexane ringis puckered into a low-energy conformation
17. The cycloalkane ring with the largest amountof ring-strainis:- a cyclooctane
- b cyclopropane
- c cyclopentane
- d cyclodecane
- e C13H26
Use the following in answering Questions 18-20Q) cis-1,2-dimethylcyclohexane R) trans-1,2-dimethylcyclohexaneS) 1,1-dimethylcyclohexane T) chair cyclohexane U) boat cyclohexane
18. Configurational isomers:- a Q and R
- b S and T
- c T and U
- d R and S
- e S and U
19. Conformational isomers:- a T and U
- b R and S
- c S and U
- d Q and R
- e S and T
20. Constitutional isomers:- a S and T
- b T and U
- c Q and R
- d R and S
- e S and U
21. A cycloalkane that can exist in aconformation containing twoaxial methyl groups [Please note; Four of the following arecyclohexanes, one is a cyclopropane.]:- a 1,1-dimethylcyclohexane
- b cis-1,2-dimethylcyclohexane
- c trans-1,2-dimethylcyclopropane
- d trans-1,3-dimethylcyclohexane
- e trans-1,4-dimethylcyclohexane
22. When the chair conformation oftrans-1,2-dimethylcyclohexaneundergoes a ring-flip, the product is:- a cis-1,2-dimethylcyclohexane
- b eclipsed-1,2-dimethylcyclohexane
- c staggered-1,2-dimethylcyclohexane
- d trans-1,2-dimethylcyclohexane
- e anti-1,2-dimethylcyclohexane
23. The total number of conformations in whichcis-1,4-dimethylcyclohexane can exist is:24. A common feature of decalin, camphor, andcholesterol is:- a the presence of a carbonyl(C=O) group on a ring-carbon
- b an axial methyl group in themorestable conformation
- c CH3/CH3elipsing in the more stable conformation
- d lack of any possibility ofgeometric isomerism
- e presence of two ormore fused rings
25. The difference between the total amount ofenergy in theproducts of a reaction and the total amount of energy in itsreactants is:- a (delta)H
- b T(delta)S
- c (delta)S
- d (delta)H-T(delta)S
- e (delta)G-T(delta)H
26. Given the following values for bonddissociation energies,Bond D (kJ/mol)H-H 436H-Cl 432Cl-Cl 243H2C=CH-H 444H2C=CH-Cl 368
the heat of the following reaction H2C=CH2 + Cl2 -----> H2C=CH-Cl + H-Clis (ignoring the algebraic sign):
- a 113
- b 117
- c 1487
- d 133
- e 269
27. The bond dissociation energy ofH2 is 436 kJ/mol. We conclude that:- a a molecule of H2releases 436 kJ/mol when it dissociates into two, infinitelyseparated hydrogen atoms
- b a molecule of H2releases436 kJ/mol when it combines with oxygen to form H2O
- c according to the equationE=mc2, the total energy content of all the matter inH2 is 436 kJ/mol
- d the formation of thecovalent bond of H-H releases 436 kJ/mol
- e 436 kJ/mol must be added to twoindependent H atoms to create an H2 molecule
28. A compound of molecular formula C10H14undergoes catalytichydrogenation but absorbs only two molar equivalents of hydrogen.How many rings does the compound have: 29. The degree of unsaturation in DDT,C14H9Cl5 is: 30. According to the Cahn-Ingold-Prelog sequencerules, the orderof priority for methyl, bromine, hydrogen, and iodinesubstituents from highest priority to lowest priority is:- a -CH3 -Br -H -I
- b -Br -I -H -CH3
- c -I -CH3 -H -Br
- d -CH3 -I -Br -H
- e -I -Br-CH3 -H
31. The E,Z-system is not applicable to:- a 1-bromo-2-methylpropene
- b 1,2-dibromoethene
- c 2-butene
- d 3,4-dibromo-3-hexene
- e 1-bromopropene
32. The alkene that releases the smallest amountof heat, per mole, when it is hydrogenated to an alkane is:- a ethene
- b 2,3-dimethyl-2-butene
- c isobutylene
- d cis-2-pentene
- e 1-decene
33. Markovnikov's Rule concerns:- a the thermodynamic stabilitiesof alkenes
- b the use of the E,Z-system fornaming geometric isomers
- c ring-flip in alkyl-substitutedcyclohexane rings
- d the products ofelectrophilic addition to alkenes
- e Gibbs free energy changes thatoccur in organic mechanisms
= THE END =
You now have the choice of: